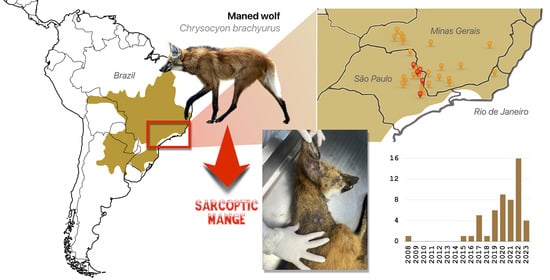
The Sarcoptic Mange in Maned Wolf (Chrysocyon brachyurus): Mapping an Emerging Disease in the Largest South American Canid
Abstract
:1. Introduction
2. Methodology
2.1. Study Design, Study Area and Study Period
2.2. Confirmed Cases (Group G1)
2.3. Suspected Cases (Group G2)
3. Results
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motta-Junior, J.C.; Talamoni, S.A.; Lombardi, J.A.; Simokomaki, K. Diet of the maned wolf, Chrysocyon brachyurus, in central Brazil. J. Zool. 1996, 240, 277–284. [Google Scholar] [CrossRef]
- Kleiman, D.G. Social Behavior of the Maned Wolf (Chrysocyon brachyurus) and Bush Dog (Speothos venaticus): A Study in Contrast. J. Mammal. 1972, 53, 791–806. [Google Scholar] [CrossRef]
- Jácomo, A.T.A.; Kashivakura, C.K.; Ferro, C.; Furtado, M.M.; Astete, S.P.; Tôrres, N.M.; Sollmann, R.; Silveira, L. Home Range and Spatial Organization of Maned Wolves in the Brazilian Grasslands. J. Mammal. 2009, 90, 150–157. [Google Scholar] [CrossRef]
- Paula, R.C.; Rodrigues, F.H.G.; Queirolo, D.; Jorge, R.P.S.; Lemos, F.G.; Rodrigues, L.A. Avaliação do estado de conservação do lobo-guará Chrysocyon brachyurus (Illiger, 1815) no Brasil. Biodivers. Bras. 2013, 3, 146–159. [Google Scholar]
- Paula, R.C.; Dematteo, K. Chrysocyon brachyurus. In The IUCN Red List of Threatened Species; 2015; Available online: https://www.iucnredlist.org/species/4819/88135664 (accessed on 1 December 2021). [CrossRef]
- Gittleman, J.L.; Funk, S.M.; MacDonald, D.W.; Wayne, R.K. (Eds.) Carnivore Conservation; Cambridge University Press: Cambridge, UK, 2001; Volume 5. [Google Scholar]
- May, J.A., Jr.; Felippe, P.A.N. Conservation Medicine. In Ecology and Conservation of the Maned Wolf—Multidisciplinary Perspectives; Consorte-McCrea, A.G., Santos, E.F., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Miller, R.S.; Farnsworth, M.L.; Malmberg, J.L. Diseases at the livestock–wildlife interface: Status, challenges, and opportunities in the United States. Prev. Vet. Med. 2013, 110, 119–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goller, K.V.; Fyumagwa, R.D.; Nikolin, V.; East, M.L.; Kilewo, M.; Speck, S.; Müller, T.; Matzke, M.; Wibbelt, B. Fatal canine distemper infection in a pack of African wild dogs in the Serengeti ecosystem, Tanzania. Vet. Microbiol. 2010, 146, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justice-Allen, A.; Clement, M.J. Effect of Canine Parvovirus and Canine Distemper Virus on the Mexican Wolf (Canis lupus baileyi) Population in the USA. J. Wildl. Dis. 2019, 55, 682–688. [Google Scholar] [CrossRef]
- Penezić, A.; Selakovic, S.; Pavlović, I.; Ćirović, D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef]
- Okulewicz, A.; Perec-Matysiak, A.; Buńkowska, K.; Hildebrand, J. Toxocara canis, Toxocara cati and Toxascaris leonina in wild and domestic carnivores. Helminthologia 2012, 49, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Criado-Fornelio, A.; Gutierrez-Garcia, L.; Rodriguez-Caabeiro, F.; Reus-Garcia, E.; Roldan-Soriano, M.; Diaz-Sanchez, M. A parasitological survey of wild red foxes (Vulpes vulpes) from the province of Guadalajara, Spain. Vet. Parasitol. 2000, 92, 245–251. [Google Scholar] [CrossRef]
- Dietz, J.M. Ecology and social organization of the maned wolf (Chrysocyon brachyurus). In Smithsonian Contributions to Zoology, n. 392; Smithsonian Institution Press: Washington, DC, USA, 1984; pp. 1–51. [Google Scholar] [CrossRef]
- Deem, S.L.; Emmons, L.H. Exposure of free-ranging maned wolves (Chrysocyon brachyurus) to infectious and parasitic disease agents in the Noël Kempff Mercado National Park, Bolivia. J. Zoo Wildl. Med. 2005, 36, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Provença, L.M. Soroprevalência de Doenças Infecciosas Caninas em Populações de Lobo-Guará (Chrysocyon brachyurus) e Cachorro-do-Mato (Cerdocyon thous) na Estação Ecológica de Águas Emendadas, DF. Master’s Thesis, Universidade de Brasilia, Brasília, Brazil, 2007. [Google Scholar]
- Vieira, F.M.; Luque, J.; Muniz-Pereira, L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008, 1721, 1–23. [Google Scholar] [CrossRef]
- Braga, R.T.; Vynne, C.; Loyola, R.D. Fauna parasitária intestinal de Chrysocyon brachyurus (lobo-guará) no Parque Nacional das Emas. Bioikos 2010, 24, 49–55. [Google Scholar]
- Mattos, P.S.R. Epidemiologia e Genética Populacional do Lobo-Guará, Chrysocyon brachyurus (Illiger, 1915) (Carnívora, Canidae) na Região Nordeste do Estado de São Paulo. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2003. [Google Scholar]
- Braga, R.T.; Vynne, C.; Corrêa, M.C.R.; Loyola, R.D. New record of Dyoctophyma renale in the maned wolf (Chrysocyon brachyrus) in the state of Goiás, Brazil. Bioikos 2010, 24, c43–c47. [Google Scholar]
- Vieira, F.M. Helmintos Parasitos de Mamíferos Carnívoros Silvestres no Município de Juiz de Fora, Zona da Mata do Estado de Minas Gerais, Brasil. Ph. D. Thesis, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Arrais, R.C.; Paula, R.C.; Martins, T.F.; Nieri-Bastos, F.A.; Marcili, A.; Labruna, M.B. Survey of ticks and tick-borne agents in maned wolves (Chrysocyon brachyurus) from a natural landscape in Brazil. Ticks Tick-borne Dis. 2021, 12, 101639. [Google Scholar] [CrossRef]
- Rodrigues, T.O.; Canelo, E.A.; Sommerfeld, S.; Moraes, F.P.; Nascimento, F.G.O.; Silva, D.M.; Lima, A.M.C.; Santos, A.L.Q. Cinomose em lobo-guará Chrysocyon brachyurus (Illiger, 1811): Relato de caso. Veterinária Notícias 2014, 20, 24. [Google Scholar]
- Vieira, E.G.; Araújo, G.V.B.; Tolomeu, A.L.G.; Cardoso, V.A.F.X.; Mendes, A.L.P.; Machado, J.P. Infecção por Dioctophyma renale com localização livre em cavidade abdominal de lobo-guará (Chrysocyon brachyurus): Relato de caso. In Proceedings of the VI Simpósio de Produção Acadêmica (SIMPAC 2014), Viçosa, Brazil, 22–28 October 2014; pp. 363–368. [Google Scholar]
- Escobar, L.E.; Carver, S.; Cross, P.C.; Rossi, L.; Almberg, E.S.; Yabsley, M.J.; Niedringhaus, K.D.; Van Wick, P.; Dominguez-Villegas, E.; Gakuya, F.; et al. Sarcoptic mange: An emerging panzootic in wildlife. Transbound. Emerg. Dis. 2022, 69, 927–942. [Google Scholar] [CrossRef]
- Astorga, F.; Carver, S.; Almberg, E.S.; Sousa, G.R.; Wingfield, K.; Niedringhaus, K.D.; Van Wick, P.; Rossi, L.; Cross, P.; Angelone, S.; et al. International meeting on sarcoptic mange in wildlife, June 2018, Blacksburg, Virginia, USA. Parasites Vectors 2018, 11, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, F. Cingulata (Tatus) e Pilosa (Preguiças e Tamanduás). In Tratado de Animais Selvagens Medicina Veterinária, 2nd ed.; Cubas, Z.S., Silva, J.C.R., Catão-Dias, J.L., Eds.; Roca: São Paulo, Brazil, 2014; Volume 1, pp. 707–722. [Google Scholar]
- Nogueira, M.F.; Cruz, T.F. Doenças da Capivara; Embrapa Pantanal: Corumbá, Brazil, 2007. [Google Scholar]
- Almeida, J.C.; Leal, C.A.S.; Melo, R.P.B.; Albuquerque, P.P.F.; Pedrosa, C.M.; Zermiani, F.C.; Farias, R.C.; Mota, R.A. Co-Infection by Sarcoptes scabiei and Microsporum gypseum in Free-Ranging Crab-Eating Fox, Cerdocyon thous (Linnaeus, 1766). Braz. Arch. Biol. Technol. 2018, 61, e18160508. [Google Scholar] [CrossRef]
- Teodoro, T.G.; Lima, P.A.; Stehling, P.C.; Junior, I.M.O.; Varaschin, M.S.; Wouters, F.; Wouters, A.T.B. Sarcoptic mange (Sarcoptes scabiei) in wild canids (Cerdocyon thous). Pesq. Vet. Bras. 2018, 38, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.S.; Costa, E.O.; Benites, N.R. Processos dermatológicos em animais silvestres: Avaliação clínica e laboratorial de dermatopatias em silvestres revela prevalência de causas parasitárias, bacterianas e fúngicas. Clin. Vet. 1997, 2, 16–19. [Google Scholar]
- Currier, R.W.; Walton, S.F.; Currie, B.J. Scabies in animals and humans: History, evolutionary perspectives, and modern clinical management. Ann. N. Y. Acad. Sci. 2012, 1230, E50–E60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Parasitologia Veterinária; Guanabara: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Zachary, J.F.; McGavin, M.D. Bases da Patologia em Veterinária, 5th ed.; Elsevier: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Pence, D.B.; Ueckermann, E. Sarcoptic mange in wildilfe. Rev. Sci. Tech. OIE 2002, 21, 385–398. [Google Scholar] [CrossRef]
- Uzai, G.J.S.; Monteiro, C.P.; Soares, R.; Silva, M.A.; Oliveira, A.R.; Santos, R.; Nunes, L. Morphological and molecular diagnosis of diseases of free-ranging crab-eating foxes (Cerdocyon thous). Arq. Bras. Med. Vet. Zoo 2021, 73, 583–588. [Google Scholar] [CrossRef]
- Verdugo, C.; Espinoza, A.; Moroni, M.; Valderrama, R.; Hernandez, C. Sarcoptic Mange in a South American Gray Fox (Chilla Fox; Lycalopex griseus), Chile. J. Wildl. Dis. 2016, 52, 738–741. [Google Scholar] [CrossRef]
- Villalba-Briones, R.; Molineros, E.B.; Monrós, J.S. First report of Sarcoptes scabiei parasitism (Sarcoptiformes: Sarcoptidae) in Lycalopes sechurae (Mammalia: Carnivora). Rev. Bras. Parasitol. Vet. 2022, 31, e005022. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.S.P.; Jorge, M.L.S.P. Carnívora—Canidae (Cachorro-do-mato, Cachorro-vinagre, Lobo-guará e Raposa-do-campo). In Tratado de Animais Selvagens Medicina Veterinária, 2nd ed.; Cubas, Z.S., Silva, J.C.R., Catão-Dias, J.L., Eds.; Roca: São Paulo, Brazil, 2014; Volume 1, pp. 764–778. [Google Scholar]
- Jorge, R.S.P.; Lima, E.S.; Lucarts, L.E.B. Sarna sarcóptica ameaçando cachorros-vinagres (Speothos venaticus) de vida livre em Nova Xavantina, MT. In Proceedings of the XXXIII Congresso Anual Sociedade de Zoológicos do Brasil, Sorocaba, Brazil, 21–25 March 2009. [Google Scholar]
- Luque, J.A.D.; Muller, H.; González, L.; Berkunsky, I. Clinical sings suggestive of mange infestation in a free-ranging maned wolf (Chrysocyon brachyurus) in the Moxos Savannahs of Bene, Bolivia. Mastozool Neotrop 2014, 21, 135–138. [Google Scholar]
- Olifiers, N.; Bianchi, R.d.C.; D’Andrea, P.S.; Mourão, G.; Gompper, M.E. Estimating age of carnivores from the Pantanal region of Brazil. Wildl. Biol. 2010, 16, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Chevallier, C.; Gauthier, G.; Berteaux, D. Age Estimation of Live Arctic Foxes Vulpes lagopus Based on Teeth Condition. Wildl. Biol. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.P.; Sharma, V.; Goyal, S.P.; Stevens, H.; Gupta, S.K. Age estimation of Tiger Panthera tigris (Linnaeus, 1758) and Lion Panthera leo (Linnaeus, 1758) (Mammalia: Carnivora: Felidae): Applicability of cementum annuli analysis method. J. Threat. Taxa 2022, 14, 21805–21810. [Google Scholar] [CrossRef]
- Emmons, L.H. Maned Wolves of Noel Kempff Mercado National Park; Smithsonian Contributions to Zoology, n. 639; Smithsonian Institution Press: Washington, DC, USA, 2012; pp. 1–35. [Google Scholar] [CrossRef] [Green Version]
- Instituto Chico Mendes de Conservação da Biodiversidade [ICMBio]. Sumário Executivo do Plano de Ação Nacional Para a Conservação do Lobo-Guará; Ministry of Environment and Climate Change: Brasília, Brazil, 2017. [Google Scholar]
- Gardiner, C.H.; Poynton, S.L. An Atlas of Metazoan Parasites in Animal Tissues; Armed Forces Institute of Pathology, American Registry of Pathology: Washington, DC, USA, 1999; ISBN 1881041492. [Google Scholar]
- Hnilica, K.A. Dermatologia de Pequenos Animais: Atlas Colorido e Guia Terapêutico, 3rd ed.; Elsevier: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Urquhart, G.M.; Armour, J.; Duncan, J.L.; Dunn, A.M.; Jennings, F.W. Parasitologia Veterinária, 2nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 1998. [Google Scholar]
- Carneiro, V.O. Ocorrência da Sarna Sarcóptica em Cães Domiciliados no Bairro Vila Verde na Cidade de Tabatinga—AM; Graduation Final Work; Universidade do Estado do Amazonas: Tabatinga, Brazil, 2019. [Google Scholar]
- Souza, C.; Azevedo, T. MapBiomas General Handbook; MapBiomas: São Paulo, Brazil, 2017; pp. 1–23. [Google Scholar]
- Conceição, L.G.; Loures, F.H. Sistema tegumentar. In Patologia Veterinária, 2nd ed.; Santos, R.L., Alessi, A.C., Eds.; Roca: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Sinclair, A.R.E.; Fryxell, J.M.; Caughley, G. Wildlife Ecology Conservation and Management, 2nd ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Haas, C.; Origgi, F.C.; Akdesir, E.; Linhares, M.B.; Giovannini, S.; Mavrot, F.; Casaubon, J.; Ryser-Degiorgis, M.-P. First detection of sarcoptic mange in free-ranging wild boar (Sus scrofa) in Switzerland. Schweiz. Arch. Tierheilkd. 2015, 157, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Origgi, F.C.; Rossi, S.; López-Olvera, J.R.; Rossi, L.; Castillo-Contreras, R.; Malmsten, A.; Dalin, A.-M.; Orusa, R.; Robetto, S.; et al. Serological survey in wild boar (Sus scrofa) in Switzerland and other European countries: Sarcoptes scabiei may be more widely distributed than previously thought. BMC Vet. Res. 2018, 14, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardells, J.; Lizana, V.; Martí-Marco, A.; Lavín, S.; Velarde, R.; Rossi, L.; Moroni, B. First description of sarcoptic mange in an Iberian hare (Lepus granatensis). Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100021. [Google Scholar] [CrossRef] [PubMed]
- de Faria, G.M.M.; Rosa, C.A.; Corrêa, G.L.C.; Puertas, F.; Jiménez, K.M.O.; Perillo, L.N.; Hufnagel, L.; Leles, B.; de Paula, R.C.; Rodrigues, F.H.G.; et al. Geographic distribution of the European hare (Lepus europaeus) in Brazil and new records of occurrence for the Cerrado and Atlantic Forest biomes. Mammalia 2016, 80, 497–505. [Google Scholar] [CrossRef]
- Instituto Brasileiro do Meio Ambiente e dos Recursos Renováveis [IBAMA]. Relatório Sobre Áreas Prioritárias Para o Manejo de Javalis: Aspectos ambientais, Socioeconômicos e Sanitários; IBAMA: Brasília, Brasil, 2019. [Google Scholar]


| Identification Groups | Individuals | Date of Record | Municipality | State | |
|---|---|---|---|---|---|
| (mm/yyyy) | |||||
| G1 | Cbr 1 | 05/2022 | Mococa | São Paulo | |
| Cbr 4 | 01/2008 | Itatiba | São Paulo | ||
| Cbr 6 | 07/2019 | Mococa | São Paulo | ||
| Cbr 7 | 04/2021 | Mococa | São Paulo | ||
| Cbr 10 | 12/2017 | Mogi-Guaçu | São Paulo | ||
| Cbr 11 | 12/2017 | Mogi-Guaçu | São Paulo | ||
| Cbr 12 | 12/2017 | Mogi-Guaçu | São Paulo | ||
| Cbr 13 | 04/2021 | São João da Boa Vista | São Paulo | ||
| Cbr 14 | 09/2018 | São José do Rio Pardo | São Paulo | ||
| Cbr 48 | 02/2023 | Cajuru | São Paulo | ||
| G2 | G2A | Cbr 2 | 06/2022 | Mococa | São Paulo |
| Cbr 3 | 08/2022 | Mococa | São Paulo | ||
| Cbr 5 | 03/2019 | Mococa | São Paulo | ||
| Cbr 8 | 04/2021 | Mococa | São Paulo | ||
| Cbr 9 | 09/2017 | Mogi-Guaçu | São Paulo | ||
| Cbr 15 | 05/2022 | Mococa | São Paulo | ||
| Cbr 16 | 09/2021 | Mococa | São Paulo | ||
| Cbr 17 | 06/2020 | Itapira | São Paulo | ||
| Cbr 18 | 04/2020 | Mogi-Guaçu | São Paulo | ||
| Cbr 19 | 11/2020 | Mogi-Guaçu | São Paulo | ||
| Cbr 20 | 10/2019 | São José do Rio Pardo | São Paulo | ||
| Cbr 21 | 03/2020 | Botelhos | Minas Gerais | ||
| G2B | Cbr 22 | 10/2022 | Itirapina | São Paulo | |
| Cbr 23 | 07/2020 | Santo Antônio do Jardim | São Paulo | ||
| Cbr 24 | 04/2022 | São Bento do Sapucaí | São Paulo | ||
| Cbr 25 | 05/2022 | São Bento do Sapucaí | São Paulo | ||
| Cbr 26 | 11/2021 | São Bento do Sapucaí | São Paulo | ||
| Cbr 27 | 12/2020 | São Carlos | São Paulo | ||
| Cbr 28 | 03/2022 | São José do Rio Pardo | São Paulo | ||
| Cbr 29 | 03/2019 | Serra Negra | São Paulo | ||
| Cbr 30 | 02/2021 | Arceburgo | Minas Gerais | ||
| Cbr 31 | 07/2022 | Botelhos | Minas Gerais | ||
| Cbr 32 | 04/2022 | Campos Altos | Minas Gerais | ||
| Cbr 33 | 03/2020 | Córrego Novo | Minas Gerais | ||
| Cbr 34 | 11/2022 | Uberlândia | Minas Gerais | ||
| Cbr 35 | 05/2016 | Jardinésia | Minas Gerais | ||
| Cbr 36 | 09/2020 | Pouso Alto | Minas Gerais | ||
| Cbr 37 | 03/2017 | Santana da Vargem | Minas Gerais | ||
| Cbr 38 | 01/2021 | São João del Rei | Minas Gerais | ||
| Cbr 39 | 01/2019 | São Gonçalo do Sapucaí | Minas Gerais | ||
| Cbr 40 | 08/2022 | Soledade de Minas | Minas Gerais | ||
| Cbr 41 | 07/2015 | Uberlândia | Minas Gerais | ||
| Cbr 42 | 07/2022 | Santo Antônio de Pádua | Rio de Janeiro | ||
| Cbr 43 | 07/2022 | Uberaba | Minas Gerais | ||
| Cbr 44 | 07/2021 | Uberaba | Minas Gerais | ||
| Cbr 45 | 12/2022 | Inconfidentes | Minas Gerais | ||
| Cbr 46 | 01/2023 | Itirapina | São Paulo | ||
| Cbr 47 | 10/2020 | Monte Alegre do Sul | São Paulo | ||
| Cbr 49 | 12/2022 | Itirapina | São Paulo | ||
| Cbr 50 | 01/2019 | Pedregulho | São Paulo | ||
| Cbr 51 | 03/2023 | Campestre | Minas Gerais | ||
| Cbr 52 | 01/2023 | Tambaú | São Paulo | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiori, F.; de Paula, R.C.; Navas-Suárez, P.E.; Boulhosa, R.L.P.; Dias, R.A. The Sarcoptic Mange in Maned Wolf (Chrysocyon brachyurus): Mapping an Emerging Disease in the Largest South American Canid. Pathogens 2023, 12, 830. https://doi.org/10.3390/pathogens12060830
Fiori F, de Paula RC, Navas-Suárez PE, Boulhosa RLP, Dias RA. The Sarcoptic Mange in Maned Wolf (Chrysocyon brachyurus): Mapping an Emerging Disease in the Largest South American Canid. Pathogens. 2023; 12(6):830. https://doi.org/10.3390/pathogens12060830
Chicago/Turabian StyleFiori, Flávia, Rogério Cunha de Paula, Pedro Enrique Navas-Suárez, Ricardo Luiz Pires Boulhosa, and Ricardo Augusto Dias. 2023. "The Sarcoptic Mange in Maned Wolf (Chrysocyon brachyurus): Mapping an Emerging Disease in the Largest South American Canid" Pathogens 12, no. 6: 830. https://doi.org/10.3390/pathogens12060830